Ozone layer depletion is one of the most serious environmental problems in today’s world. Ozone layer absorbs about 97-99% of the harmful ultraviolet (UV) radiation from the Sun and is vital for existence of life on Earth. Depletion of this layer will leave planet Earth defenseless against the harmful effects of UV radiation. In this general knowledge article, we will discuss about the basics of ozone and ozone layer depletion.
What is Ozone?
Ozone is a highly reactive gas that occurs naturally in the atmosphere. Ozone is made up of three oxygen atoms (O3). It occurs in both the upper atmosphere (the stratosphere) and lower atmosphere (the troposphere) of Earth. Depending on it’s location in the atmosphere, ozone has either good or bad effects on the life on Earth.
In the upper atmosphere, ozone is formed naturally by chemical reactions involving solar ultraviolet radiation (sunlight) and oxygen molecules. It protects earth from incoming UV radiation from Sun. In the lower atmosphere near the Earth’s surface (the troposphere), ozone is formed as a result of reactions between sunlight and air pollutants, mainly nitrogen oxides (from vehicle exhaust, combustion processes etc) and volatile organic compounds (VOC) such as petroleum products. At ground level, high concentrations of this ‘bad ozone’ is toxic to both plants and animals.

What is Ozone Layer?
Ozone layer is a layer in upper atmosphere where ozone is found in highest concentrations. Also known as Ozonosphere, it forms a thick layer in stratosphere. The ozonosphere is located at altitudes of 15-35 km (9 to 22 miles) above the surface of the earth. The average concentration of ozone is around 0.6 PPM (parts per million). The highest concentrations of ozone occur at altitudes from 26 to 28 km (16 to 17 miles) in the tropics and from 12 to 20 km (7 to 12 miles) towards the poles. The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. The thickness of the ozone layer changes by season and location.
Why Ozone Layer is Necessary?
Ozone layer is important because it absorbs harmful ultraviolet radiation from the Sun, which is highly energetic and damages both plants and animals exposed to it. In atmosphere UV radiations with higher wavelengths are absorbed by diatomic oxygen. But radiation between 240 – 290 nm are not absorbed by the diatomic oxygen molecules. Those wavelengths are absorbed by ozone. Absorption of UV radiations by the ozone layer is important for life to flourish on planet Earth.
Ozone Layer Depletion
Ozone Layer Depletion is the process in which the ozone layer becomes thinner due to the destruction of ozone by several man-made substances in the atmosphere. Ozone depletion occurs when destruction of the stratospheric ozone is more than the production of the molecule. During the last several decades, human activities have resulted in considerable reduction in the ozone layer of the atmosphere. Scientists have observed reduction in stratospheric ozone since early 1970s. In some areas the depletion process is so worse that a hole appears in the ozone layer. Technically, ozone hole is not a ‘hole’, but a region of exceptionally depleted ozone in the stratosphere. Usually ozone holes form over the Poles during the onset of spring seasons. One of the largest such hole appears annually over Antarctica between September and November.
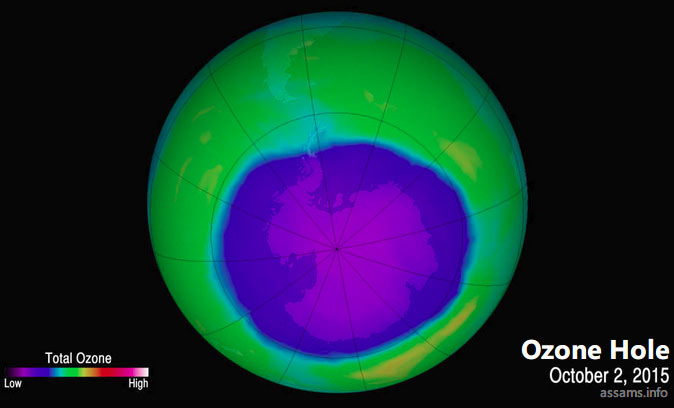
Causes of Ozone layer depletion:
Causes of ozone layer depletion can be categorized into two broad categories – natural causes and man-made causes.
- Natural causes: Certain natural phenomena such as Sun-spots and stratospheric winds affects ozone layer. But those causes attributes to only about 1-2% depletion of the ozone layer and the effects are also temporary. Major volcanic eruptions may also contribute towards ozone depletion.
- Man-made causes: The main cause of depletion of ozone is the release of chlorine and bromine from man-made chemicals such as chlorofluorocarbons (CFCs) and other Ozone depleting substances (ODS). One chlorine can destroy 100,000 ozone molecule. And a Bromine atom is believed to be 40 times more destructive than a chlorine molecule.
Ozone depleting substances (ODS): These are the chemicals that contribute to depletion of the ozone layer. The major ODS are: chlorofluorocarbons (CFC), Methyl chloroform (CH3CCl3), Halons, Carbon tetrachloride (CCl4), Hydro-chlorofluorocarbons (HCFCs), Hydrobromofluorocarbons (HBFCs) and Methyl bromide (CH3Br). These are used in various products and processes including freezers, air cooling, dry-cleaning agents, aerosols, adhesives, fire extinguishers etc.
Effects of Ozone layer depletion:
Some of the directs effects of ozone layer depletion are outlined below:
- Effects on Human: Exposure to UV radiation will result in increased cases of skin cancers, sunburns and premature aging of the skin, cataracts, blindness and other eye diseases. It will also weaken human immune system.
- Effects on Plants: UV radiation reduces leaf size and area available for photosynthesis. So, stunting growth and reduction in dry matter production occurs. Quality of certain fruits will deteriorate. Majority of the field crops will result in poor yield.
- Effects on Marine Ecosystems: UV radiation can penetrate up to 20 meter deep in water. So, plankton and other light depending marine organisms will suffer from cell damages. Plankton is the foundation of aquatic food chains. Decreases in plankton means disruption in both fresh and saltwater food chains.
- Effects on Animals: UV radiation exposure may cause eye and skin cancers in animals. As life cycle of plants will change, there will be disruption in food chain.
References:
- Environmental effects of ozone depletion and its interactions with climate change: Progress Report 2015; United Nations Environmental Programme, Environmental Effects Assessment Panel.
- Scientific Assessment of Ozone Depletion: 2014; World Meteorological Organization.
- Bharucha, Erach. Textbook of Environmental Studies for Undergraduate Courses.
- United Nations Environmental Programme, www.unep.org.

Liked it. Would be better if found in Assamese.